PASI-75 RESPONSE @ 16 WEEKS: OTEZLA
DEMONSTRATED A
STATISTICALLY
SIGNIFICANT IMPROVEMENT
VS. PLACEBO1,3

Adapted from the OTEZLA Product Monograph, 2020, and Papp K, et al., 2015.
PASI-75 EFFICACY REMAINED STABLE UP
TO WEEK 32
(WEEKS 16–32 WERE AN
UNCONTROLLED MAINTENANCE PHASE;
2° ENDPOINT)1,5
What percentage of your patients have moderate-to-severe PsO?

* Full analysis set; last observation carried forward.
PASI: MEAN PERCENT CHANGE
(IMPROVEMENT) IN PASI
SCORES FROM
BASELINE OVER 16 WEEKS (2° ENDPOINT)3,6

| Week | 0 | 2 | 4 | 8 | 12 | 16 |
|---|---|---|---|---|---|---|
| OTEZLA 30 mg BID, n | 562 | 547 | 541 | 525 | 508 | 501 |
| Placebo, n | 282 | 273 | 270 | 264 | 249 | 247 |
Adapted from Papp K, et al., 2015, and data on file.

* Full analysis set; as observed.
CHANGE IN PRURITUS VAS SCORE @
16 WEEKS:
STATISTICALLY SIGNIFICANT
DIFFERENCE SHOWN WITH OTEZLA
TREATMENT VS. PLACEBO (2° ENDPOINT)3
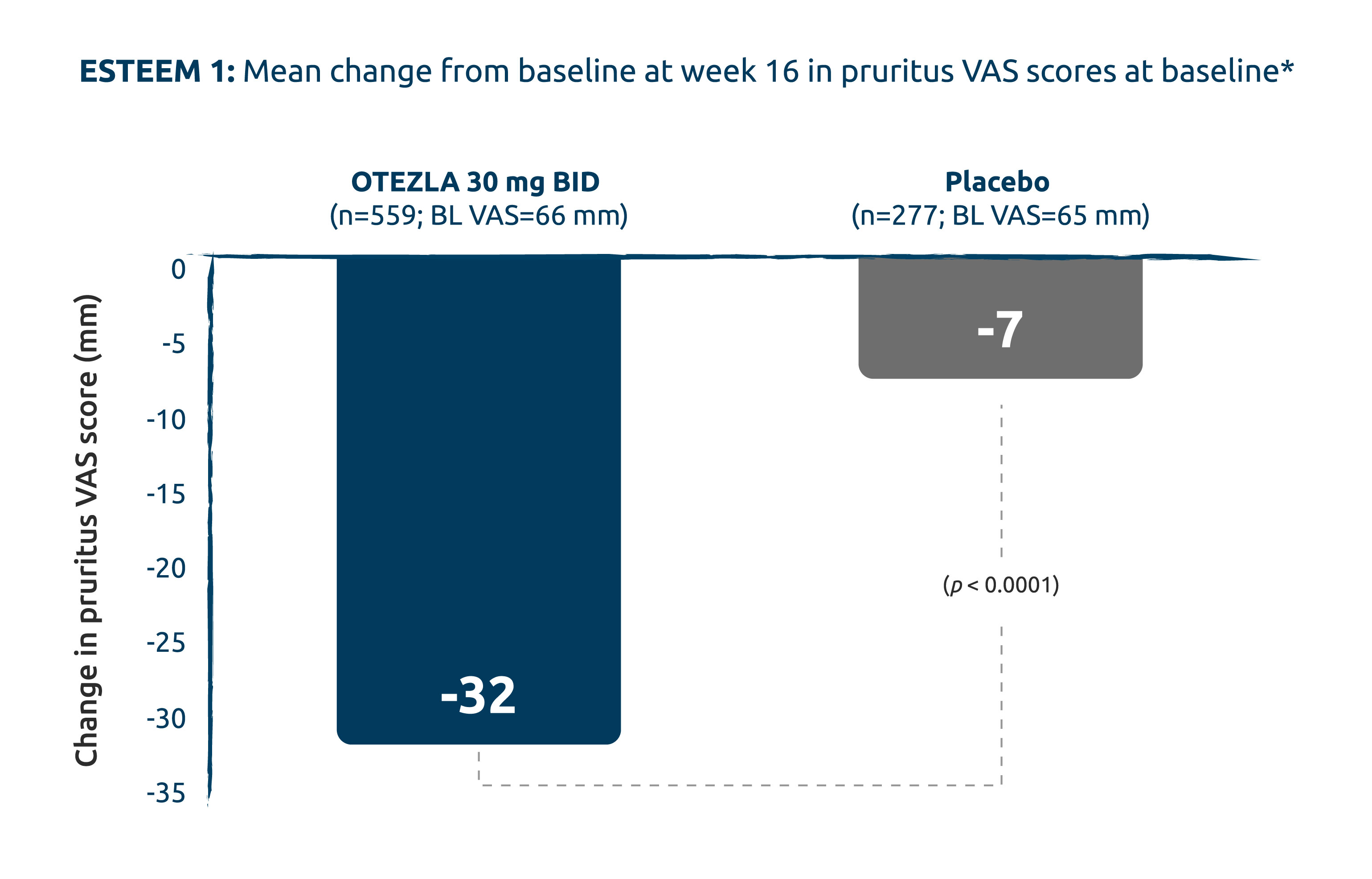
* Pruritus VAS ranges from 0–100. Higher scores correspond to worse pruritus. Patients with a baseline value and ≥ 1 post-baseline value are included.1
Adapted from Papp K, et al., 2015.3
What proportion of your patients with moderate-to-severe plaque psoriasis present with pruritus?

BID, twice daily; BL, baseline; PASI, Psoriasis Area and Severity Index; PsO, psoriasis; VAS, visual analog score.