AUC FOR THE NUMBER OF ORAL ULCERS @ 12 WEEKS: OTEZLA DEMONSTRATED A RAPID, STATISTICALLY SIGNIFICANT IMPROVEMENT VS. PLACEBO1,12
Primary endpoint: Taking OTEZLA 30 mg twice daily resulted in significant improvement in oral ulcers vs. placebo, as demonstrated by the AUC for the number of oral ulcers from baseline through 12 weeks (129.54 vs. 222.14, respectively; LS mean; p < 0.0001).
PATIENTS EXPERIENCED STATISTICALLY SIGNIFICANTLY LOWER DAILY AVERAGE NUMBER OF ORAL ULCERS WITH OTEZLA VS. WITH PLACEBO (2° ENDPOINT)1,12

Adapted from the OTEZLA Product Monograph, 2020.1
OTEZLA maintained oral ulcer improvements through week 64 in patients originally randomized to OTEZLA 30 mg BID and who remained on treatment (75 of 104; open label and uncontrolled from weeks 12–64).1†
* Mean baseline oral ulcer counts were OTEZLA: 4.2, placebo: 3.9.1
† Data as observed. Among the 104 patients who were originally randomized to OTEZLA 30 mg BID, 75 patients remained on treatment.1
COMPLETE ORAL ULCER RESPONSE (ORAL ULCER–FREE) @ 12 WEEKS: STATISTICALLY SIGNIFICANTLY MORE PATIENTS WERE ORAL ULCER–FREE WITH OTEZLA VS. WITH PLACEBO (2° ENDPOINT)1
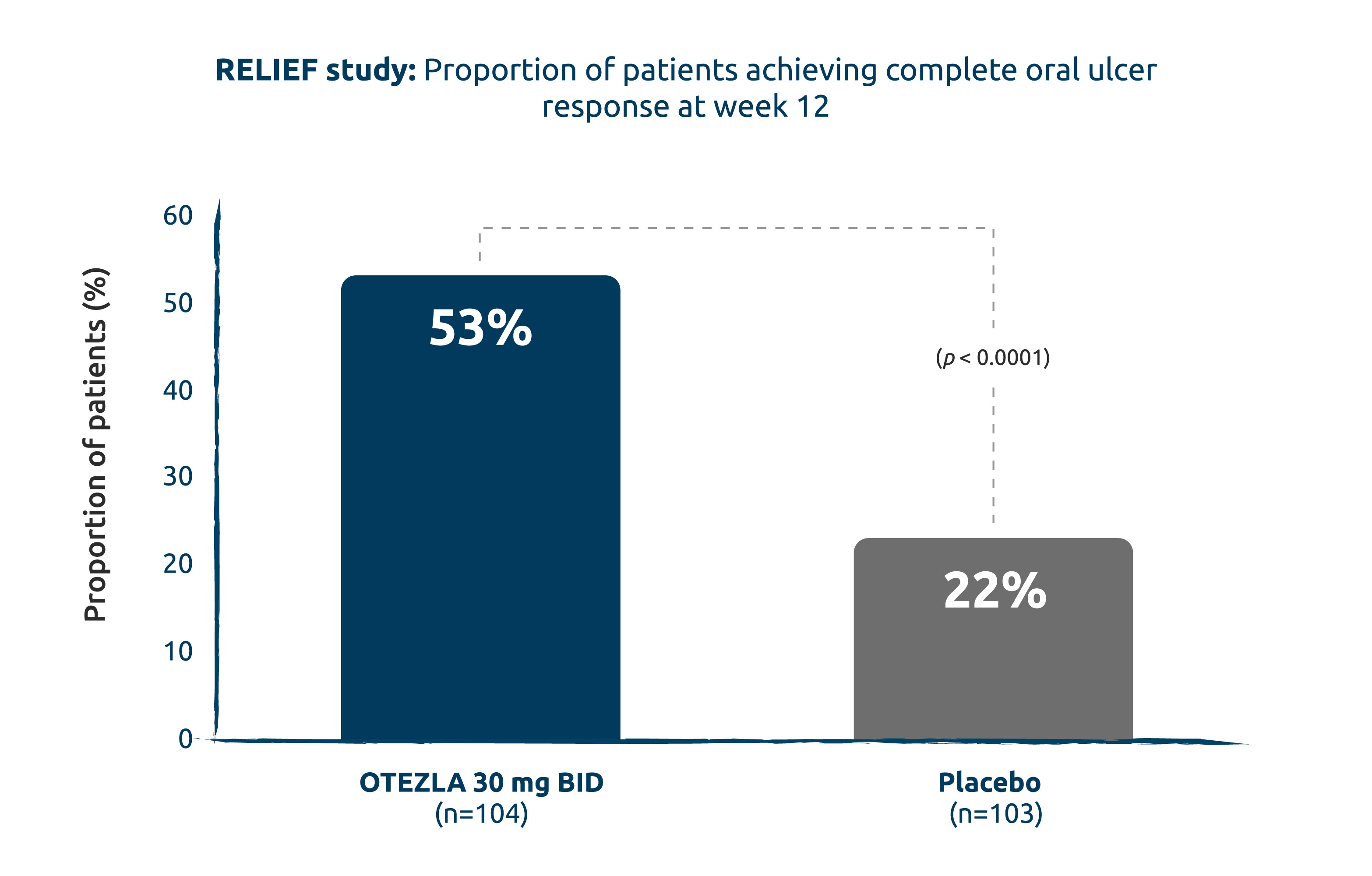
Intent-to-treat population; non-responder imputation.
Adapted from the OTEZLA Product Monograph, 2020.1
2.4x more patients were oral ulcer–free with OTEZLA vs. with placebo.1
RESOLUTION OF ORAL ULCERS (ORAL ULCER–FREE) WITH MAINTENANCE OF RESOLUTION: OTEZLA DEMONSTRATED A STATISTICALLY SIGNIFICANT DIFFERENCE VS. PLACEBO (2° ENDPOINT)1
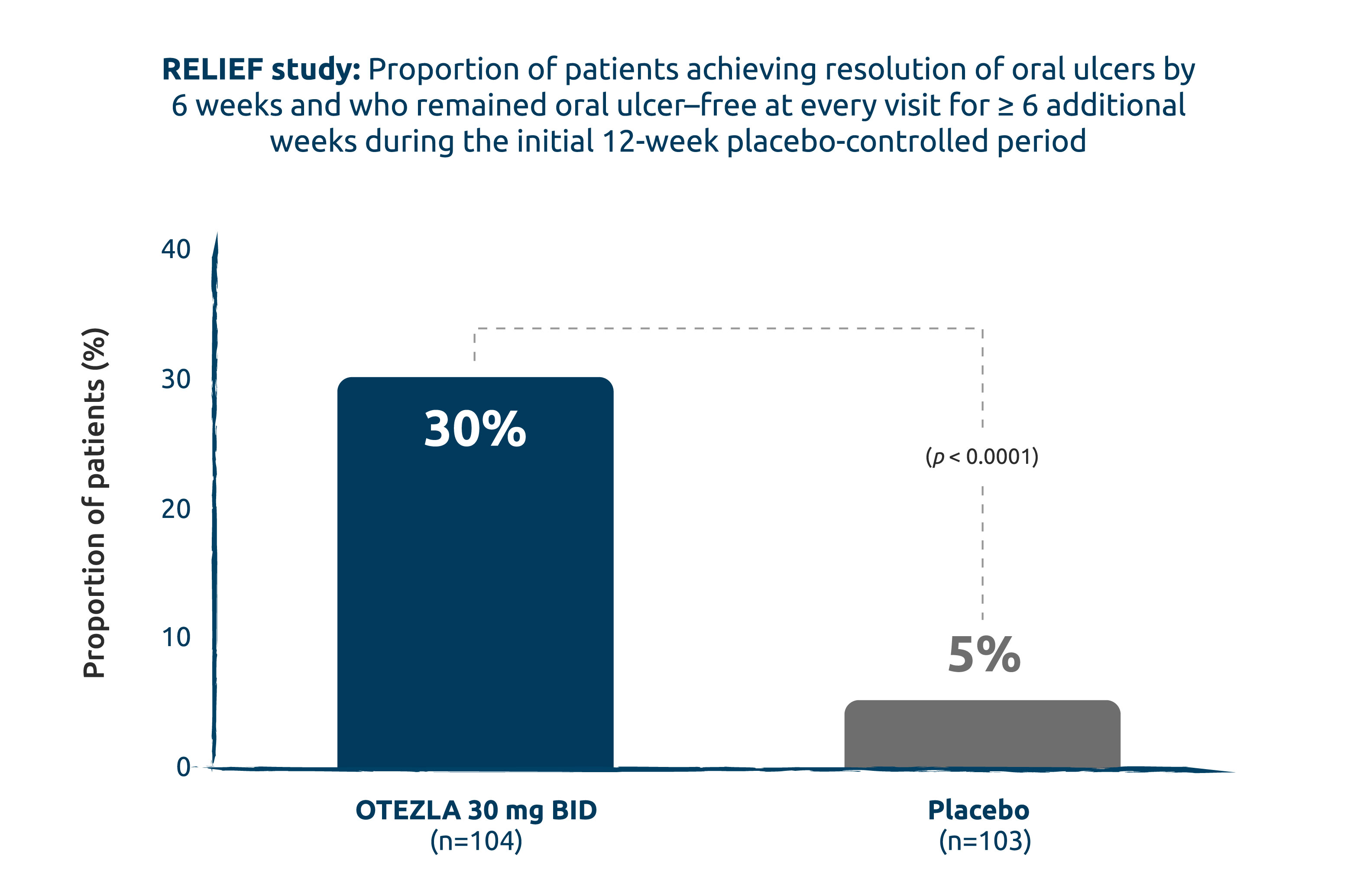
Intent-to-treat population.
Adapted from the OTEZLA Product Monograph, 2020.1
6x more patients achieved resolution of oral ulcers (oral ulcer–free) by week 6 and maintained response for 6 additional weeks vs. placebo.1
CHANGE IN ORAL ULCER VAS PAIN SCORE @ 12 WEEKS: OTEZLA STATISTICALLY SIGNIFICANTLY REDUCED PAIN ASSOCIATED WITH ORAL ULCERS VS. PLACEBO (2° ENDPOINT)1,12
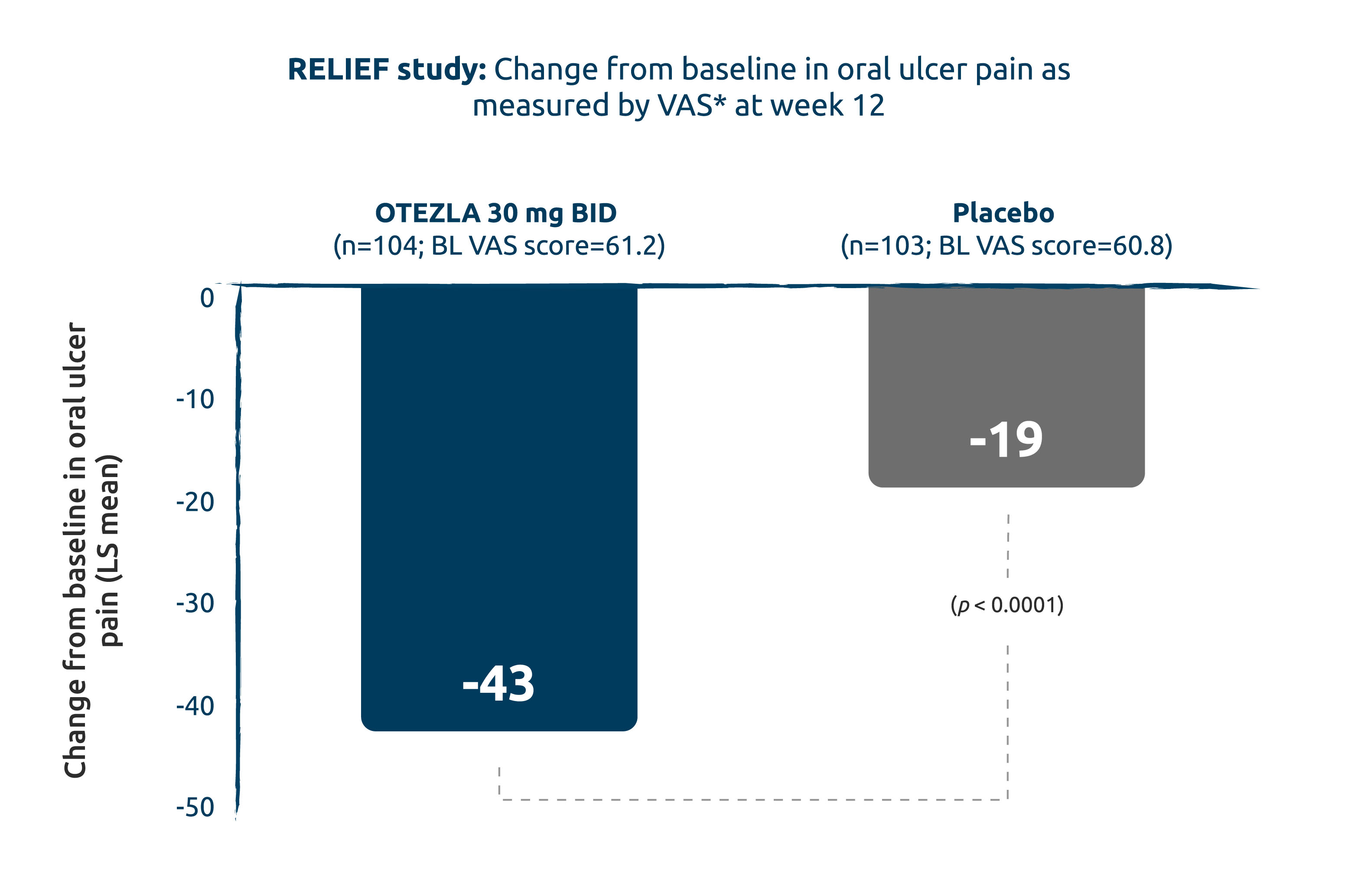
Intent-to-treat population; mixed-effects model for repeated measures.
Adapted from the OTEZLA Product Monograph, 2020, and Hatemi G, et al., 2019.1,12
2.3x greater reduction of pain associated with oral ulcers vs. placebo.1
OTEZLA maintained reduction of oral ulcer pain through week 64 in patients originally randomized to OTEZLA 30 mg BID and who remained on treatment (75 of 104; open label and uncontrolled from weeks 12–64).1†

* Oral ulcer pain was assessed on a 100 mm VAS with 0=no pain and 100=worst possible pain.1
† Data as observed. Among the 104 patients who were originally randomized to OTEZLA 30 mg BID, 75 patients remained on treatment.1
BID, twice daily; BL, baseline; LS, least squares; SE, standard error; VAS, visual analog scale.